A Brief Explanation of the Data Available for Viewing

The data being displayed represent many of the key measures of water and habitat quality for living resources such as fish, crabs and oysters. This information is being collected to guide the restoration of the Chesapeake Bay, its tributaries, Coastal Bays, and Maryland lakes by identifying specific problems and evaluating the success of management initiatives. Like the atmosphere we are more familiar with, water is a dynamic environment. Just as our weather - temperature, humidity and precipitation - varies from hour to hour and from season to season, aquatic habitats – using measures such as dissolved oxygen, chlorophyll (algae) and water clarity - are constantly changing as well. In order to understand our impacts on the Chesapeake Bay, tributaries, and lakes, and long-term trends in water and habitat quality, we must be able to measure and account for these short-term and seasonal dynamics.
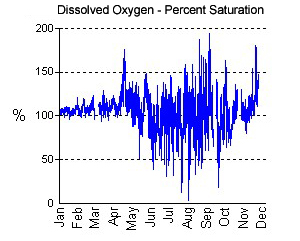
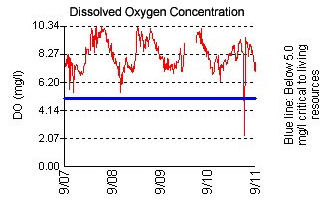
Dissolved Oxygen
The amount of oxygen dissolved in Chesapeake Bay, tributary, or lake waters is probably the single most important
measure of habitat quality; without oxygen, all of the living resources familiar
to us perish. Dissolved oxygen (DO) is measured as a concentration (mg/l –
milligrams per liter). When DO concentrations drop below 5 mg/l, more
sensitive organisms, such as fish, become stressed, especially if exposed to
these conditions for prolonged periods. Bottom-dwelling organisms such as worms
are usually more tolerant, and some species can survive at levels down to 1
mg/l in some cases. However, most aquatic organisms will not survive exposure to water with less than 1 mg/l of dissolved oxygen for more than a few hours.
|
|
In most cases, the DO graphs from the continuous monitoring stations show daily variations, with peaks in late
afternoon and minimums at dawn. These peaks are due to the production of oxygen by algae (measured by chlorophyll) during the daytime and the consumption of oxygen at night by algae and other organisms in the water and bottom sediments. These daily swings can be quite large when there are algae blooms fueled by nutrient pollution, and they often result in fish kills when oxygen levels drop to around 1 mg/l or less.
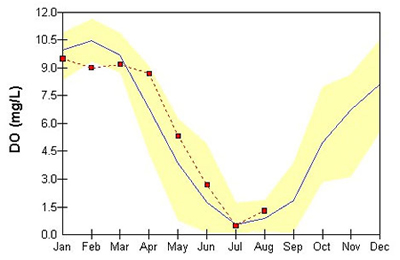
The deeper areas of the Chesapeake Bay, tributaries, and lakes tend to show very low oxygen conditions during the summer as can be seen in the monthly data for the mainstem and the lower sections of the larger tributaries. A depression in DO concentrations is natural in these deep waters due to restricted mixing, but the problem has been made worse by nutrient-fueled algae blooms that sink and decompose in these areas.
|
Algal
Blooms
Algal blooms, can be very damaging to aquatic habitats since they can drive DO concentrations
to very low levels. Excess algae, usually caused by an excess of nutrients which
stimulate their growth, can also make the water cloudy, or turbid, blocking
the light needed by bay
grasses, or submerged aquatic vegetation (SAV). These damaging algae blooms,
which can also produce toxins in some cases, are collectively known as harmful
algal blooms. The amount of algae in the water is measured as chlorophyll
concentration (ug/l – micrograms per liter). Chlorophyll is the main chemical
responsible for photosynthesis in plants, the process by which sunlight is converted
into food energy. There are no hard and fast rules as to what constitutes a
harmful concentration of chlorophyll but as a general guide, above 50 ug/l represents
a significant bloom and above 100 ug/l represents a severe bloom. Some research
suggests that harmful effects can occur at chlorophyll concentrations as low
as 15 ug/l.
Turbidity
Secchi depth is a measure of the clarity, or turbidity of the water. Secchi
depth is measured using a circular plate, called a Secchi disk, which is divided
into quarters painted alternately black and white.
 The Secchi disk is lowered into the water until it is no longer visible,
and that depth is measured. Secchi depth values that are high indicate clearer
water, and low Secchi depths indicate high turbidity. Turbid waters typically
appear cloudy and have high concentrations of total suspended solids (TSS), thereby
allowing less light to penetrate through the water. As described above, increased
turbidity is often due to excessive algal growth. However, turbidity can also
increase due to land run-off and shore-line erosion, pollution, resuspension of
bottom sediments, dredging operations, or during high periods of fresh-water input
from rivers and streams. Turbidity is typically high in areas known as turbidity
maximum zones, which occur at the edge of salt wedges where freshwater and saltwater
mixing occurs. Highly turbid waters, or waters with low secchi depth, tend to
prevent the growth of bay
grasses, which provide DO to the water column and critical habitat for many
fish and invertebrate species.
The Secchi disk is lowered into the water until it is no longer visible,
and that depth is measured. Secchi depth values that are high indicate clearer
water, and low Secchi depths indicate high turbidity. Turbid waters typically
appear cloudy and have high concentrations of total suspended solids (TSS), thereby
allowing less light to penetrate through the water. As described above, increased
turbidity is often due to excessive algal growth. However, turbidity can also
increase due to land run-off and shore-line erosion, pollution, resuspension of
bottom sediments, dredging operations, or during high periods of fresh-water input
from rivers and streams. Turbidity is typically high in areas known as turbidity
maximum zones, which occur at the edge of salt wedges where freshwater and saltwater
mixing occurs. Highly turbid waters, or waters with low secchi depth, tend to
prevent the growth of bay
grasses, which provide DO to the water column and critical habitat for many
fish and invertebrate species.
Water clarity can also be measured more accurately
using a transmissometer, which records turbidity values in Nephelometric Turbidity
Units (NTUs). Turbidity values over a threshold of 15 NTUs are normally considered
to be detrimental to bay
grass growth. Increased turbidity can also lead to decreased fish health by
increasing susceptibility to infectious diseases through increased stress, and
reducing the ability of fish's gills to extract DO from the water. High areas
of turbidity can also cause the silting over of benthic
organisms, the equivalent to being buried alive. However, this silting is
more common in areas of dredging where large quantities of sediment are disturbed
over short periods of time.
Salinity
The concentration of salt, or salinity, is a function of the mixing of freshwater
from Chesapeake Bay tributaries with ocean waters, which contain approximately
32 ppt (parts per thousand) salinity. In any given location, salinity can vary
greatly depending upon river flow, being low during high flows and high during
droughts. Most of the Bay’s living
resources are adapted to these large swings in salinity, but extreme floods
or droughts can lead to stressful conditions. For example, prolonged extreme
low salinity can lead to mortality of clams and other benthic
organisms. Conversely, extended periods of high salinity brought on during
periods of drought
can lead to mass oyster mortality, by increasing the distribution and virulence
of oyster parasites. Extended periods of high salinity can also force fish that
prefer lower salinities, such as yellow perch, out of river mainstems and up
into headwater creeks. These areas often have large algae blooms and low DO
conditions, which can be stressful or even lethal to fish. Freshwater species
of bay
grasses, such as wild celery, cannot move and may be stressed or killed
by the rising salinities brought on by a drought.
Temperature
Temperature, like salinity, undergoes wide variations seasonally, although it
is much less variable and much more predictable than any other habitat measurement
displayed here. This can be seen by looking at the historical range for the
long-term stations for any given month. This relative stability is due to the
heat retaining properties of water, which make it much more resistant to temperature
changes than our atmosphere.
pH, in simple terms, is a chemical measure of whether or not something is an acid or a base. It is measured on a log scale of 0 to 14, with each unit representing a ten-fold change. A pH of 7
is considered neutral and a range of 5.5 to 8.5 is usually tolerated by most aquatic organisms. Lower pHs are sometimes seen in fresher waters
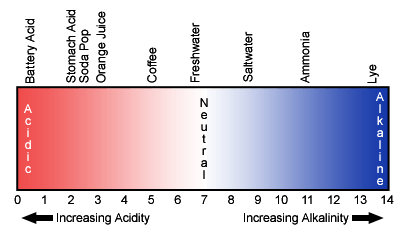 due to acid precipitation or even naturally-occurring organic acids, which can be found in areas with extensive marshes. High pHs can occur during algae blooms due to chemical processes associated with photosynthesis. Moderate to higher salinities usually “buffer” pH in the 7 to 8 range, so most of the more extreme values will be found in low salinity situations.
due to acid precipitation or even naturally-occurring organic acids, which can be found in areas with extensive marshes. High pHs can occur during algae blooms due to chemical processes associated with photosynthesis. Moderate to higher salinities usually “buffer” pH in the 7 to 8 range, so most of the more extreme values will be found in low salinity situations.
There are many interactions among the various water and habitat quality measurements described above, some of which have already been mentioned. The displays of time series for the various stations provide an excellent opportunity to explore some of these relationships.